Physician scientists are physicians (MDs or DOs with or without additional degrees) who devote regular components of their professional effort to seeking new knowledge about health, disease, or delivery of patient care through research. While all physicians receive training in medical science, physician scientists are trained to conduct independent scientific investigations in the laboratory, clinic or other settings.
A physician scientist's in-depth clinical knowledge of human health and disease and scientific investigation and analysis skills make them uniquely resourceful. Physician scientists are well prepared to:
- Detect new threats to human health
- Develop potential new therapies, treatments, or means of prevention
- Communicate knowledgeably across disciplines
- Lead scientific teams or organizations
- Guide important policy decisions, such as drug approval

Aspiring physician scientists are supported through several programs by the School of Medicine Office of Research. Please see below and contact us at somrd@hs.uci.edu for more information.
The School of Medicine Office of Research is excited to announce the launch of the Physician Scientist Collective (PSC) at UCI, a community of like-minded researchers dedicated to advancing scientific discovery and improving patient outcomes. The PSC aims to provide a platform for physician scientists to come together, learn, collaborate, and advance their careers.
One of the main programs offered by the PSC is the monthly seminar series. These seminars will include visiting speakers who will discuss their research and career paths. Other seminars will be educational and cover a variety of topics, including study design, tissue acquisition and analysis, mentorship, and grantsmanship. These seminars are designed to provide physician scientists with the knowledge and skills they need to conduct high-quality research and advance their careers.
Moreover, the PSC provides opportunities for networking and collaboration. The organization also offers mentorship and career development resources to help physician scientists achieve their professional goals. The Physician Scientist Collective is an excellent resource for physician scientists who want to connect with their peers, learn new skills, and advance their careers.
Please see our Events calendar below for a list of upcoming PSC Distinguished Speaker Series events.
The UC Irvine School of Medicine's new interdisciplinary research showcase provides a platform for residents and fellows to present their innovative research, learn from their peers, and connect with mentors and institutional leaders. This program fosters collaboration across departments, provides opportunities to advance medical discovery, and strengthens our physician-scientist community.
Note to Departments: This showcase serves as a complement to department-specific research days.
For more information, please contact the Research Development Unit at somrd@hs.uci.edu.
The School of Medicine Office of Research is pleased to offer the Physician Scientist Pathways Certificate Course. This course will prepare physician scientists by enabling them to combine their clinical knowledge with skills in scientific investigation. The School of Medicine strives to advance individual and population health and this fundamental certificate course will equip physician scientists with the basic knowledge required to address their research questions. This will allow UCI to deliver top-level care to our patients, as we will have physicians able to leverage ongoing breakthroughs while advancing research in their fields.
Director: Anand Ganesan, MD, PhD
Program Manager: Mary Frances Ypma-Wong, PhD
Target Applicant
Residents, fellows, and junior faculty physicians interested in pursuing a career path that includes basic, translational, clinical and/or epidemiology research. This course is designed to not only provide a basic level of knowledge but for attendees to develop an individual development plan for further research training.
Program Structure
This course will run for 6 weeks, 2 hours/week. It is a hybrid course, which includes 3 in-person sessions and 3 asynchronous online sessions which are completed independently. There is no tuition fee.
- Week 1: Physician Scientist Research Pathways and Opportunities | in-person | on September 18, 4:00-6:00pm on the School of Medicine campus in Irvine.
- Week 2: Tools of Basic and Translational Research
- Week 3: Basics of Biostatistics and Epidemiology
- Week 4: Mentors and Collaborators| in-person | October 9, 4:00-6:00pm at UCIMC
- Week 5: Translational Tissue Models
- Week 6: Designing a fundable research question and training plan| in-person | on October 23, 4:00-6:00pm on the School of Medicine campus in Irvine.This course includes weekly assessments as required by the SOM certificate compliance policies.
Program Objectives
- Understand the different types of biomedical research pathways and the types of training that are needed for each pathway.
- Understand the process of formulating a research question and how to search for funding opportunities (through PIVOT, etc.)
- Develop an individualized training plan to obtain the necessary research skills for further career development.
- Understand how the latest research and clinical tools can be applied.
- Obtain a fundamental understanding of the grant application process and what is required to assemble a grant application that could be funded.
Application Requirements
- Complete the application by the September 6th. This includes:
- One-page letter of intent which outlines research interests and rationale/need for additional training.
- Letter of support from the department chair, division chief, or program director indicating that the candidate will be provided time to attend in-person activities.
- Individual commitment to attend all three in-person sessions.
- In-person sessions are 4:00-6:00pm on Sept. 18, Oct. 9 and Oct 23. See the application for additional details.
- You will receive a notice regarding your status (accepted/not accepted) into the program.
Check back in late summer to apply for the Fall 2025 Pathways course.
Have questions?
The Program
The 2024 Physician Scientist Training Program is a two-year structured program providing physician scientists with intensive mentorship and opportunities to apply for their first independent, federally funded awards (K08, K23) or other equivalent external career development awards. This program is supported by the School of Medicine and is administered by the School of Medicine Research Development Unit (RDU) with oversight by the School's Associate Dean of Physician Scientist Development and the Vice Deans of Clinical and Basic Research.
The 2024 program will run from June 2024 through May 2026.
PSTP leverages the wide range of resources at UCI, including those provided by the School of Medicine Offices of Research and of Academic Affairs, Institute of Clinical and Translational Science (ICTS), and the Chao Family Comprehensive Cancer Center (CFCCC). This is a competitive, two-year program that incorporates the following elements:
- Formal and informal mentoring.
- Access to resources to facilitate appropriate study design, collection of pilot data, and preparation and submission of competitive grant applications.
- Instruction in and structured support for navigating NIH and other extramural funding sources, grant writing and review, and grants management with the goal of successfully submitting independent applications.
In addition, participants will also be able to tailor training according to their individual career and research goals. RDU staff will work with physicians to ensure access to mentors and resources.
Eligibility
This program is open to physician scientists (MD, DO, or MD, PhD) who meet the following eligibility requirements:
- Holds a junior faculty position (typically instructor or assistant professor)
- U.S. citizen or permanent resident
- Has not been a principal investigator or equivalent on any of the following:
- NIH R01 (or equivalent)
- NIH career development award (e.g. K08, K23)
- Equivalent Public Health Service or VA research grants/career awards.
- Able to devote at least 25% effort for the duration of the program to research. For those admitted to the program, certification of time commitment from a participant’s department chair or division chief will be required prior to program launch.
- Proposed research falls within the clinical & translational science research spectrum
- Has at least one scientific mentor with aligned research expertise, extramural funding, and a strong commitment to guide and support the applicant’s proposed career development, research program and grant-writing goals.
- Candidates may and are encouraged to identify more than one mentor, i.e., a mentoring team if deemed advantageous for providing expert advice in all aspects of their research and career development.
Program Requirements & Milestones
At least 25% of a physician scientist’s full-time professional effort must be devoted to research activities. The participant must be committed to attending programmatic events, communicating individualized training needs to School of Medicine RDU staff, and reaching the milestones outlined below:
- Attend orientation and workshops for grant funding, grant writing, and grants management.
- Join the Physician Scientist Collective and attend monthly events.
- Attend and prepare for all scheduled meetings with the scientific mentor mentoring team at least once a month.
- Develop a grant writing schedule by the end of the first 3 months of the program and generate and circulate drafts of grant components for review by the end of the first 12 months.
- Report on progress in the program and meet with the PSTP review committee at 6, 12, 18 and 24 months.
- Submit an NIH K08 or K23 application by June 2026.
To Apply
A complete application includes the following. The program is not currently accepting applications.
- 1-2 page career statement and professional development plan for pursuing a research career in clinical and translational science. This must include a list of potential mentors.
- One page description of research interests and specific aims that would lead to a grant submission within 2 years.
- NIH biosketch.
- Letter of Commitment from the Department or Division Chair acknowledging candidate would be provided at least 25% protected time for research activities.
For inquiries, please contact the Research Development Unit at somrd@hs.uci.edu.
The Dean’s NIH K Scholar Program is a one-year program intended to provide time and support so that the scholar can optimize and resubmit a scored NIH K application with the goal of a funded NIH K award. There are specific requirements for the K Scholar, who will be supported by the School of Medicine Dean’s Office and their department.
For more information on the K Scholar Program, contact the RDU at somrd@hs.uci.edu.
K Society provides comprehensive support for physician-scientists with NIH-K and VA CDA awards transitioning to independent research funding (R01/VA Merit). Members receive grantsmanship advice, peer and faculty proposal review, and career development resources.
This School of Medicine program is administered by the Research Development Unit (RDU) and overseen by the Associate Dean of Physician Scientist Development and the Vice Dean of Clinical Research. To join K Society, contact the RDU at somrd@hs.uci.edu.
The Program
The ACCTITP aims to promote equitable delivery of advanced therapeutics through educating community physicians, advanced practice providers (APPs) and pharmacists on scientific progress in regenerative medicine and training them in cell and gene therapy clinical methodology.
- The ACCTITP will be offered as a certificate course through UCI School of Medicine Office of Medical Education.
- The lectures will be offered as weekly two-hour lecture sessions for 20 weeks (Thursdays from 6:00-8:00 pm over Zoom) with ten half-day clinical research observational sessions in the UCI AC clinics and inpatient units.
Target Audience
Priority will be given to physicians interested in clinical research careers with an emphasis on the delivery of clinical trials in community settings, including our AC REAL sites. We plan to enroll between four and six candidates every year.
To Apply
Check the ACCITITP website regarding the next application cycle.
If you have any questions about the program, please contact Dr. Monique Williams (monique.williams@uci.edu).
Read more about the ACCTITP.
2025 Clinical Trialist Training Program (CTTP) Announcement
The Clinical Trialist Training Program (CTTP) is an initiative by the UCI School of Medicine Office of Research aimed at increasing the number of School of Medicine faculty who can serve as principal investigators on externally funded clinical trials. This is achieved through a two-year partnership program with a senior investigator already running clinical trials, along with educational resources to provide foundational knowledge in clinical trial administration.
Eligibility:
- Interest in serving as a principal investigator on clinical trials
- Any faculty appointment in the School of Medicine with an active clinical practice
- Commitment from department chair or division chief to dedicate 10% FTE to clinical trial activities
- Commitment from a senior investigator already engaged in clinical trials to serve as a mentor and support the mentee in developing an independent clinical trial portfolio (incentive will be provided to the senior investigator for their commitment)
Program Milestones:
End of Year One
- Completion of required training. This training consists of weekly two-hour lecture sessions for 11 weeks, starting in January 2025 (Thursdays from 6-8 p.m. over Zoom).
- Serve as a sub-investigator on at least one to two additional clinical trials.
- Presentation of portfolio-building strategies, including the number of clinical trials in the pipeline.
- Alpha Clinic Clinical Trials Investigator Training Program (ACCTITP) Integration:
- As a CTTP mentee, you will automatically be enrolled in the first 11 weeks of the ACCTITP. This includes 19 lectures covering essential clinical trial knowledge for PIs and sub-PIs. After completing these initial 11 weeks, you have the option to continue and complete the full ACCTITP certificate program, which involves additional coursework and clinical requirements focused on cell and gene therapy clinical trials. The first 11 weeks (19 lectures) are included in your CTTP participation.
- If you choose to complete the full ACCTITP certification, the cost would be $3,750 (50% of the full tuition).
- The complete ACCTITP provides a comprehensive certificate in clinical trial management.
- If you want to pursue the full ACCTITP certification or have any questions, please contact Monique Williams at moniqutw@hs.uci.edu.
End of Year Two
- Serve as the primary UCI investigator on two new clinical trials (e.g., national cooperative group, federal, state, industry or foundation/philanthropy sponsored).
- Presentation of the present and future portfolio (e.g., number of active clinical trials, number of patients enrolled, future strategies, including the number of clinical trials in the pipeline).
Online Curriculum:
- Introduction to Clinical Trials - Focus on Regenerative Medicine
- Investigational New Drug/New Therapy and Clinical Trial Registration
- Study Planning, Feasibility and Preparation
- Institutional Review Board and Informed Consent
- Diversity, Equity and Inclusion in Clinical Trial Populations
- Clinical Research Compliance and Safety in Clinical Trials
- Study Oversight, Hiring and Managing Clinical Research Teams
- Ethical Issues Among Vulnerable Populations
- Regulations Governing Clinical Trials and Electronic Medical Records
- Contractual and Financial Considerations and Research Billing
- Research Protocol Writing and Statistical Principles
Support:
- The UCI School of Medicine Office of Research will provide the funds to support the 11 weeks of training sessions provided via the ACCTITP program.
- Trainees will receive funding for travel and registration to attend clinical trial-focused workshops, conferences and specialized training courses. This support enables them to enhance their expertise, stay current with best practices and develop professional networks for conducting high-quality clinical research.
- Mentors are provided compensation to be disbursed upon the successful completion of the program’s milestones.
To Apply:
Applications for the 2025-2027 CTTP are due by Monday, November 18, 2024. The application includes:
- Letter of interest (1 page): Briefly describe your interest in participation, area of clinical practice and populations you can enroll and which senior investigator you would like to work with.
- Letter of support from mentor, including the mentor qualifications (e.g., experience in actively conducting clinical trials, involvement in clinical trial design)
- Letter of support from the Department Chair
- CV or NIH biosketch
Please submit your application and supporting documents through the CTTP Application Portal.
Direct any questions to the UCI School of Medicine Research Development Unit.
The overview below shows funding sources accessible at different stages of a Physician Scientist’s career development and training programs that cater to unique educational needs. The timeline denotes the duration of research typically required at each phase of their journey, particularly focusing on the critical K and R stages of a Physician Scientist’s career path.
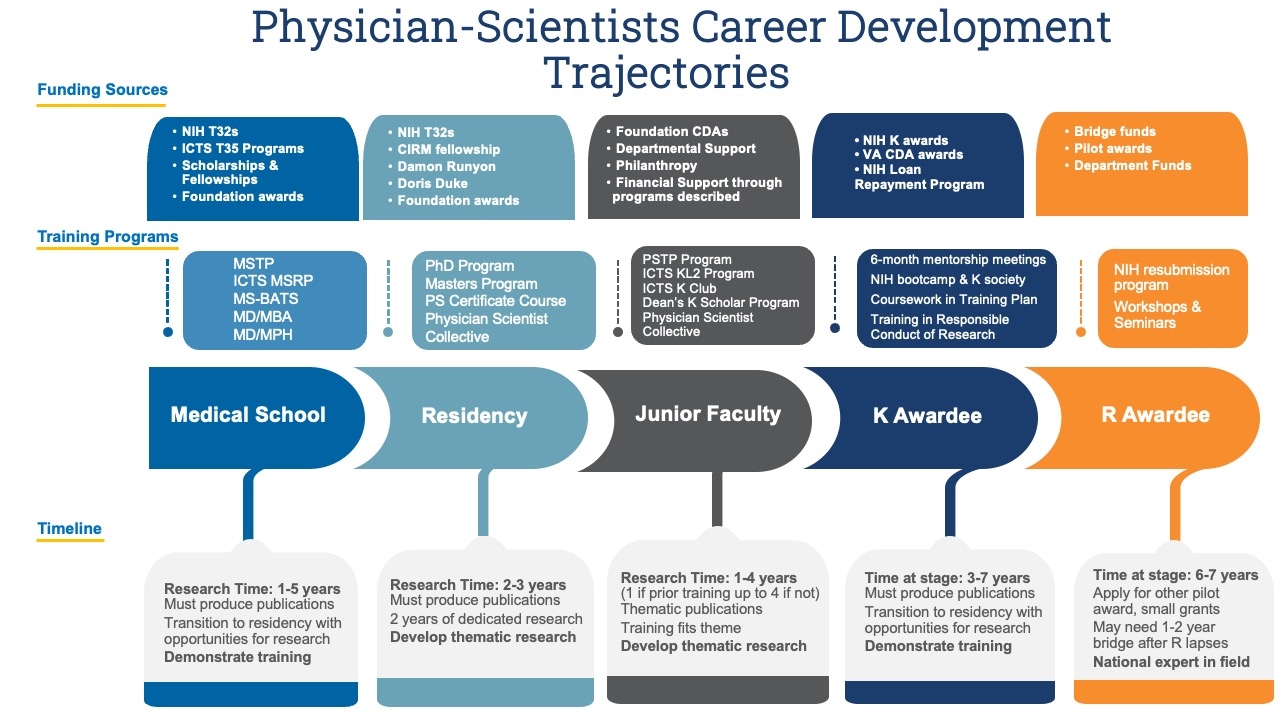
Medical School
- Research Time: 1-5 years
- Must produce publications
- Transition to residency with opportunities for research
- Demonstrate training
- Training Programs
- MSTP
- MS-BATS
- MD/MBA
- MD/MPH
- Funding sources
- NIH T32s
- MSRP
- Scholarships & Fellowships
- Foundations awards
Residency
- Research Time: 2-3 years
- Must produce publications
- 2 years of dedicated research
- Develop thematic research
- Training Programs
- PhD Programs
- Masters Program
- PS Certificate Course
- Physician Scientist Collective
- Funding sources
- NIH T32s
- CIRM fellowship
- Damon Runyon
- Doris Duke
- Foundation awards
Junior Faculty
- Research Time: 1-4 years (1 if prior training up to 4 if not)
- Thematic publications
- Training fits theme
- Develop thematic research
- Training Programs
- PSTP Program
- Dean’s K Scholar Program
- Physician Scientist Collective
- Funding sources
- Foundations CDAs
- Departmental Support
- Philanthropy
- Financial Suppor through programs described
K Awardee
- Research Time: 3-7 years
- Must produce publications
- Transition to residency with opportunities for research
- Demonstrate training
- Training Programs
- 6-month mentorship meetings
- NIH bootcamp & K society
- Coursework in Training Plan
- Training in Responsible Conduct of Research
- Funding sources
- NIH K awards
- VA CDA awards
R Awardee
- Research Time: 6-7 years
- Apply for other pilot awards, small grants
- May need a 1-2 year bridge after R lapses
- National expert in field
- Training Programs
- NIH resubmission program
- Workshops & Seminars
- Funding sources
- Bridge funds
- Pilot awards
- Department funds
Attention — School of Medicine Department Chairs & Staff
The School of Medicine Office of Research understands the challenges that may come with recruiting and hiring physician scientists to your department. We've created a comprehensive guide for hiring physician scientists.
Email Mary Frances Ypma-Wong, Research Development Manager, for the guide.
UCI Training Grants that include training for medical residents and fellows.
At UCI, we take pride in our diverse training programs designed to foster the next generation of physician scientists. Principal Investigators and trainees are encouraged to contact the respective PD/PIs listed below to inquire if there is an available training opportunity. View a complete list of UCI Training grants.
Training in the Neurobiology of Aging: 2T32AG000096-41
- PIs: Craig Stark, PhD & Andrea Tenner, PhD
- Clinical Trainees: Position for one resident/fellow postdoc
- Project Period: 09/01/82 to 04/30/2029
Interdisciplinary Training Program in Hearing Research: 5T32DC010775-14
- PIs: Raju Metherate, PhD
- Clinical Trainees: There are two postdoc positions each year, one or both can be filled by Residents or PhDs.
- Project Period: 07/01/10 – 06/30/25
A Training Program for Interdisciplinary Cancer Research: 5T32CA009054-43
- PIs: David Fruman, PhD & Aimee Edinger, VMD/PhD
- Clinical Trainees: There are two positions for postdocs holding PhD or MD-PhD degrees; appointments of MD-PhD postdocs occurs during the research fellowship phase.
- Project Period: 07/01/21 – 06/30/26
Training in Translational ADRD Neuroscience (TITAN): 5T32AG073088-03
- PIs: Joshua Grill, PhD & Elizabeth Head, PhD
- Clinical Trainees: Positions for one to two neurology, neuropathology, psychiatry, geriatrics, or nursing residents and postdocs.
- Project Period: 09/01/21 – 08/31/26
Interdisciplinary Training Program in Skin Biology: 5T32AR080622-02
- PIs: Bogi Andersen, MD & Anand Ganesan, MD/PhD
- Clinical Trainees: There are three openings for graduate students and one opening for either a PhD postdoc or a Clinical Trainee doing research.
- Project Period: 05/01/22 – 04/30/27
CIRM Scholars Comprehensive Research Training Program
- PIs: Peter Donovan, PhD
- Clinical Trainees: Supports two clinical fellows at any time for a 2-year period each.
- Project Period: 12/1/21 – 11/30/26
The UCI Physician Scientist Collective (PSC) is a platform and community for physician scientists to learn, collaborate and advance their careers. We offer programs and events tailored to the needs of physician scientists at different stages of their careers. These events occur throughout the academic year (September to June).
Bimonthly Seminar Series
These seminars feature distinguished speakers from various medical disciplines at academic institutions around the country who will share their experiences and insights on physician scientist careers.
Bimonthly Educational Seminars
We have designed educational seminars in collaboration with UCI Research Centers to develop the research skills of physician scientists on campus. These seminars will provide a forum for physician scientists to learn about new techniques and methods that may be useful in their research projects.
Previous Events
See our archive of past events and learn about the diverse topics we've previously covered.
