UCI-led study could usher in new paradigm for drug discovery
Source: UCI School of Medicine
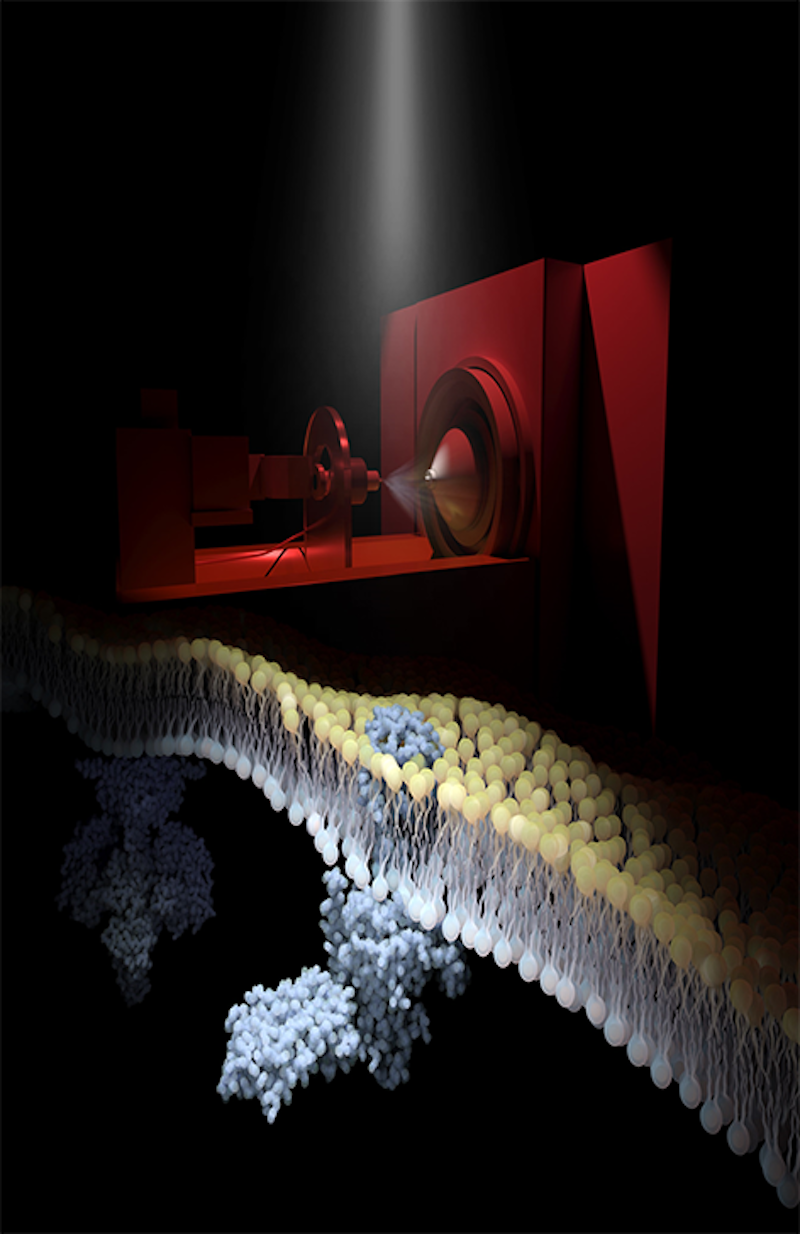
Rhodopsin, the light sensor of the mammalian visual system, is embedded in the disc membranes of photoreceptor cells. The image above shows an electrospray mass spectrometer, in a darkened, red-lit room, being used to generate ionized fragments of native disc membranes. Shown is rhodopsin activated by light while embedded in its disc membrane causing it to interact with transducin, which then signals to downstream phosphodiesterase 6 (background). Following instantaneous flashes of light, synchronized with recordings on the mass spectrometer, the research team has monitored real-time signal progression across native disc membranes. In capturing this signaling cascade they have documented roles for lipids and ligands in rhodopsin signaling, highlighting new opportunities for discovery of drug targets in the native environment.
UCI School of Medicine
Irvine, Calif., April 6, 2022 — In a new study at University of California, Irvine, researchers have revealed the impact of native lipids on rhodopsin signaling and regeneration, which may usher in a new paradigm for discovery of drugs that target G protein-coupled receptors (GPCRs).
GPCRs are cell surface receptors that respond to a variety of stimuli to activate signaling pathways across cell membranes. All GPCRs are membrane bound and have rarely been studied in their native membrane environments. Recent progress has yielded atomic structures of key intermediates and roles for lipids in in mediating the signaling. However, capturing signaling events of a wild-type receptor in real-time, across a native membrane to its downstream effectors, has remained elusive until now. These receptors by far represent the largest class of drug targets, and a vast number of approved drugs modulate their functions.
In this new study published today in Nature, titled, “Capturing a rhodopsin receptor signaling cascade across a native membrane,” the researchers, using mass spectrometry, probed the archetype class A GPCR, rhodopsin, directly in fragments of native disc membranes. They monitored real-time photoconversion of dark-adapted rhodopsin to opsin, delineating the stepwise isomerization of retinal and hydrolysis of the retinal-opsin adduct, further discovering that the reaction is significantly slower in its natural membrane environment than in artificial detergent micelles.
“Human diseases, ranging from cancer to cardiovascular diseases to blindness, are all highly impacted by the function of GPCRs. In addition to quantitative analysis of the signaling function, this novel technology, for the first time, has enabled direct detection of new potential targets of therapeutic value for the visual system, within the native membranes. I am convinced that analogous work will be done on many other GPCR systems, ” explained Krzysztof Palczewski, PhD, Donald Bren Professor of Ophthalmology at the UCI School of Medicine and co-corresponding author.
Considering the lipids ejected with rhodopsin from the membrane fragments in the mass spectrometer, researchers were able to demonstrate that opsin can be regenerated in the membranes through photoisomerized retinal-lipid conjugates, and to obtain evidence for increased association of rhodopsin with unsaturated long-chain phosphatidylcholine during signal transduction.
The team also captured the secondary steps of the signaling cascade following rhodopsin activation. Monitoring light activation of transducin (Gt), and dissociation of guanosine diphosphate (GDP) to generate intermediate apo trimeric G protein, they observed Gta.GTP subunits interacting with phosphodiesterase 6 (PDE6), found in cone and rod photoreceptor cells, which hydrolyzes the second messenger molecule cyclic guanosine monophosphate (cGMP).
“By applying rhodopsin-targeting compounds, we have shown how they either stimulate or dampen signaling via the rhodopsin-opsin and transducin signaling pathways,” said Palczewski. “Using instantaneous flashes of light, synchronized with recordings on a mass spectrometer, we were able to capture the signaling cascade and demonstrate roles for lipids and ligands in rhodopsin signaling. This work highlights new opportunities for drug discovery in native environments and may lead to a new way to investigate membrane-bound receptor pharmacology.”
